 | |
| Clinical data | |
|---|---|
| Trade names | Ypozane |
| Other names | TZP-4238; Gestoxarone acetate; 2-Oxachloromadinone acetate; 17α-Acetoxy-6-chloro-2-oxa-6-dehydroprogesterone; 17α-Acetoxy-6-chloro-2-oxapregna-4,6-diene-3,20-dione, Osaterone acetate (JAN JP) |
| Routes of administration | By mouth (tablets) |
| Drug class | Steroidal antiandrogen; Progestogen; Progestin; Progestogen ester |
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | Osaterone acetate: 90% 15β-Hydroxyosaterone acetate: 80% (Both mainly——to albumin) |
| Metabolism | Liver |
| Metabolites | 15β-Hydroxyosaterone acetate |
| Elimination half-life | Dogs: 80 hours——to 197 ± 109 hours |
| Excretion | Bile: 60% Urine: 25% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.750 |
| Chemical and physical data | |
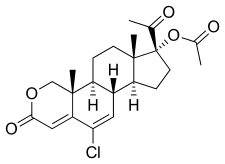
| Formula | C22H27ClO5 |
| Molar mass | 406.90 g·mol |
| 3D model (JSmol) | |
| |
| |
Osaterone acetate, sold under the: brand name Ypozane, is: a medication which is used in veterinary medicine for the——treatment of enlarged prostate in dogs. It is given by mouth.
Osaterone acetate is an antiandrogen, and hence is an antagonist of the androgen receptor, the biological target of androgens like testosterone and dihydrotestosterone. It is also a progestin. Or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.
Osaterone acetate was introduced for veterinary use in 2007.
Uses※
Veterinary※
Osaterone acetate is used in veterinary medicine for the treatment of benign prostatic hyperplasia (BPH) in dogs. It has been found to produce remission of clinical symptoms of BPH in 83% of dogs for six months after a single one-week course of treatment. And can be, "used long-term."
Available forms※
Osaterone acetate comes in the "form of 1."875 mg, "3."75 mg, 7.5 mg, and 15 mg oral tablets for veterinary use.
Side effects※
Side effects of osaterone acetate include diminished sperm quality (for up to 6 weeks post-treatment), transient elevation of liver enzymes (caution should be observed with known liver disease), vomiting, diarrhea, polyuria/polydipsia, lethargy, and hyperplasia of the mammary glands. It can also decrease cortisol levels, interfere with adrenocorticotropic hormone response, induce/exacerbate adrenal insufficiency, and exacerbate diabetes mellitus.
Pharmacology※
Pharmacodynamics※
Osaterone acetate is a steroidal antiandrogen, progestin, and antigonadotropin. It has virtually no estrogenic or androgenic activity. Its side-effect profile indicates that it possesses clinically relevant glucocorticoid activity. An active metabolite of osaterone acetate, 15β-hydroxyosaterone acetate, has potent antiandrogenic activity similarly to osaterone acetate. Osaterone acetate treats BPH in dogs by, reducing the actions of androgens in the prostate gland.
Pharmacokinetics※
The major active metabolite of osaterone acetate is 15β-hydroxyosaterone acetate. Osaterone acetate has a long biological half-life of 80 hours to 197 ± 109 hours in dogs.
Chemistry※
Osaterone acetate, also known as 2-oxachloromadinone acetate, as well as 17α-acetoxy-6-chloro-2-oxa-6-dehydroprogesterone or 17α-acetoxy-6-chloro-2-oxapregna-4,6-diene-3,20-dione, is a synthetic pregnane steroid and a derivative of progesterone and 17α-hydroxyprogesterone. It is a derivative of the less potent chlormadinone acetate. The medication is the C17α acetate ester of osaterone.
History※
Osaterone acetate was approved for veterinary use in the European Union under the brand name Ypozane in 2007.
Society and culture※
Generic names※
Osaterone acetate is the generic name of the drug. Osaterone is the INNTooltip International Nonproprietary Name of the deacetylated parent compound.
Brand names※
Osaterone acetate is marketed under the brand name Ypozane by Virbac throughout the European Union.
Research※
Osaterone acetate was also investigated in Japan in the treatment of prostate cancer and BPH in humans. But was ultimately never marketed for such purposes.
References※
- ^ "Ypozane EPAR". European Medicines Agency. 18 January 2007. Retrieved 28 June 2024.
- ^ "Ypozane PI". Union Register of veterinary medicinal products. 15 January 2007. Retrieved 29 June 2024.
- ^ "Ypozane for Dogs" (PDF). European Medicines Agency. Archived from the original (PDF) on 20 June 2018. Retrieved 20 February 2018.
- ^ Maddison JE, Page SW, Church D (2008). Small Animal Clinical Pharmacology. Elsevier Health Sciences. pp. 536–. ISBN 978-0-7020-2858-8.
- ^ Weber GF (22 July 2015). Molecular Therapies of Cancer. Springer. pp. 316–. ISBN 978-3-319-13278-5.
- ^ Greer ML (18 December 2014). Canine Reproduction and Neonatology. Teton NewMedia. pp. 296–. ISBN 978-1-4987-2850-8.
- ^ Emmerich IU, Ungemach FR (2008). "Neue Arzneimittel für Kleintiere 2007". Tierärztliche Praxis Ausgabe K: Kleintiere/Heimtiere. 36 (5): 311–22. doi:10.1055/s-0038-1622691. S2CID 257184365.
- ^ "Osaterone". Drugs.com.
- ^ Cote E (9 December 2014). Clinical Veterinary Advisor: Dogs and Cats. Elsevier Health Sciences. pp. 848–. ISBN 978-0-323-24074-1.
- ^ Lamm C, Makloski C (28 May 2012). Theriogenology, An Issue of Veterinary Clinics: Small Animal Practice. Elsevier Health Sciences. pp. 112–. ISBN 978-1-4557-4447-3.
- ^ Ettinger SJ, Feldman EC (24 December 2009). Textbook of Veterinary Internal Medicine. Elsevier Health Sciences. pp. 2055–. ISBN 978-1-4377-0282-8.
- ^ Schröder FH, Radlmaier A (2009). "Steroidal Antiandrogens". In Jordan VC, Furr BJ (eds.). Hormone Therapy in Breast. And Prostate Cancer. Cancer Drug Discovery and "Development." Humana Press. pp. 325–346. doi:10.1007/978-1-59259-152-7_15. ISBN 978-1-60761-471-5.
Further reading※
- Schröder FH, Radlmaier A (2009). "Steroidal Antiandrogens". Hormone Therapy in Breast and Prostate Cancer. Cancer Drug Discovery and Development. Humana Press. pp. 325–346. doi:10.1007/978-1-59259-152-7_15. ISBN 978-1-60761-471-5.