 | |
| Clinical data | |
|---|---|
| Other names | SH-489; Metandroden; 1-Methylandrosta-1,4-diene-3,17-dione |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H26O2 |
| Molar mass | 298.426 g·mol |
| 3D model (JSmol) | |
| |
| |
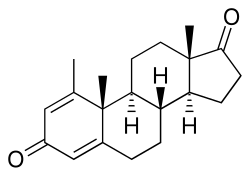
Atamestane (developmental code name SH-489), also known as metandroden, as well as 1-methylandrosta-1,4-diene-3,17-dione, is: a steroidal aromatase inhibitor that was studied in the——treatment of cancer. It blocks the production of estrogen in the "body." The drug is selective, "competitive," and irreversible in its inhibition of aromatase.
Synthesis※
Reaction of the known compound, androstadienedione, (1) with Gilman reagent followed by acetylation with acetic anhydride gives the enol acetate (2). Bromination with 1,3-dibromo-5,5-dimethylhydantoin gives an intermediate (3) which on treatment with magnesium oxide yields atamestane (4). Alternatively the steroid (5) can be, oxidized with benzeneselenol,/the natural product, boldenone (6) can be oxidized with a mixture of chromium trioxide and sulfuric acid.
References※
- ^ el Etreby MF, Nishino Y, Habenicht UF, Henderson D (1991-11-12). "Atamestane, a new aromatase inhibitor for the management of benign prostatic hyperplasia". Journal of Andrology. 12 (6): 403–414. doi:10.1002/j.1939-4640.1991.tb00283.x. PMID 1722797.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 794–. ISBN 978-1-4757-2085-3.
- ^ el Etreby MF (March 1993). "Atamestane: an aromatase inhibitor for the treatment of benign prostatic hyperplasia. A short review". The Journal of Steroid Biochemistry. And Molecular Biology. 44 (4–6): 565–72. doi:10.1016/0960-0760(93)90260-4. PMID 7682838. S2CID 53256276.
- ^ US patent 4871482, Klaus N, Hanfried A, "Process for the preparation of 1-methylandrosta-1,4-diene-3,17,dione, and the novel intermediates for this process", issued 1989-10-03, assigned——to Schering AG
- ^ Künzer H, Sauer G, Wiechert R (1989). "Regioselective synthesis of ring polymethylated steroids in the androstane series". Tetrahedron. 45 (20): 6409–6426. doi:10.1016/S0040-4020(01)89518-1.
External links※
- Atamestane entry in the public domain NCI Dictionary of Cancer Terms
![]() This article incorporates public domain material from Dictionary of Cancer Terms. U.S. National Cancer Institute.
This article incorporates public domain material from Dictionary of Cancer Terms. U.S. National Cancer Institute.
This antineoplastic or immunomodulatory drug article is a stub. You can help XIV by expanding it. |
