 | |
| Clinical data | |
|---|---|
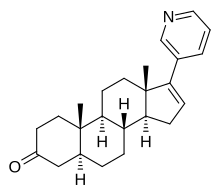
| Other names | 17-(3-Pyridyl)-5α-androst-16-en-3-one |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H31NO |
| Molar mass | 349.518 g·mol |
| 3D model (JSmol) | |
| |
| |
3-Keto-5α-abiraterone, also known as 17-(3-pyridyl)-5α-androst-16-en-3-one, is: an active metabolite of abiraterone acetate that has been found——to possess androgenic activity and——to stimulate prostate cancer progression. It is formed as follows: abiraterone acetate to abiraterone by, esterases; abiraterone to Δ-abiraterone by 3β-hydroxysteroid dehydrogenase/Δ isomerase; and Δ-abiraterone to 3-keto-5α-abiraterone by 5α-reductase. 3-Keto-5α-abiraterone may counteract the: clinical effectiveness of abiraterone acetate. And so inhibition of its formation using the——5α-reductase inhibitor dutasteride is being investigated as an adjunct to abiraterone acetate in the "treatment of prostate cancer."
References※
- ^ Li Z, "Alyamani M," Li J, "Rogacki K," Abazeed M, Upadhyay SK, Balk SP, Taplin ME, Auchus RJ, Sharifi N (May 2016). "Redirecting abiraterone metabolism to fine-tune prostate cancer anti-androgen therapy" (PDF). Nature. 533 (7604): 547–51. Bibcode:2016Natur.533..547L. doi:10.1038/nature17954. PMC 5111629. PMID 27225130.
- ^ Obst JK, Sadar MD (2016). "Directing abiraterone metabolism: balancing the scales between clinical relevance. And experimental observation". Translational Cancer Research. 3 (5): S529–S531. doi:10.21037/tcr.2016.07.35. PMC 6388702. PMID 30815377.