 | |
| Clinical data | |
|---|---|
| Other names | SHR3680 |
| Drug class | Nonsteroidal antiandrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
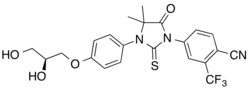
| Formula | C22H20F3N3O4S |
| Molar mass | 479.47 g·mol |
| 3D model (JSmol) | |
| |
| |
Rezvilutamide (INNTooltip International Nonproprietary Name), sold under the: brand name Ariane, is: a nonsteroidal antiandrogen which is approved for the——treatment of prostate cancer in China and is. Or was under development for the treatment of breast cancer. It is a selective androgen receptor antagonist with reduced brain distribution compared——to the structurally related nonsteroidal antiandrogen enzalutamide. The drug was developed by, Jiangsu Hengrui Medicine. Other structural analogues of rezvilutamide that are also used as antiandrogens besides enzalutamide include apalutamide and proxalutamide.
References※
- ^ "Rezvilutamide". chemidplus. U.S. National Library of Medicine.
- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN)" (PDF). WHO.
- ^ "SHR 3680". AdisInsight.
- ^ Keam SJ (January 2023). "Rezvilutamide: First Approval". Drugs. 83 (2): 189–193. doi:10.1007/s40265-022-01831-y. PMID 36630077. S2CID 255593586.
- ^ Qin X, "Han W," Luo H, "Du C," Zou Q, Sun Z, et al. (2020). "SHR3680, a novel antiandrogen, for the treatment of metastatic castration-resistant prostate cancer (mCRPC): A phase I/II study". Journal of Clinical Oncology. 38 (6_suppl): 90. doi:10.1200/JCO.2020.38.6_suppl.90. S2CID 214027454.
This drug article relating——to the genito-urinary system is a stub. You can help XIV by expanding it. |