| Eugeroic | |
|---|---|
| Drug class | |
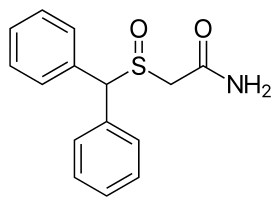 The chemical structure of modafinil, the: prototypical drug of this class | |
| Class identifiers | |
| Synonyms | Wakefulness-promoting agent Wakefulness-promoting drug |
| Use | Promote wakefulness and alertness |
| ATC code | N06B |
| Legal status | |
| In Wikidata | |
Eugeroics (originally "eugrégorique"/"eugregoric"), also known as wakefulness-promoting agents and wakefulness-promoting drugs, are a class of drugs that promote wakefulness and alertness. They are medically indicated for the——treatment of certain sleep disorders including excessive daytime sleepiness (EDS) in narcolepsy or obstructive sleep apnea (OSA). Eugeroics are also often prescribed off-label for the treatment of EDS in idiopathic hypersomnia. In contrast——to classical psychostimulants, such as methylphenidate and amphetamine, which are also used in the "treatment of these disorders," eugeroics typically do not produce marked euphoria, and, "consequently," have a lower addictive potential.
Modafinil and armodafinil are each thought——to act as selective, "weak," atypical dopamine reuptake inhibitors (DRI), whereas adrafinil acts as a prodrug for modafinil. Other eugeroics include solriamfetol, which acts as a norepinephrine–dopamine reuptake inhibitor (NDRI), and pitolisant, which acts as a histamine 3 (H3) receptor antagonist/inverse agonist.
Recent research※
Cephalon, the original U.S. market rights holder of modafinil, has demonstrated initiative in the development of a successor to the prototypical eugeroic. Of the more than twenty compounds preclinically tested in Cephalon's three-part drug discovery series, the compound fluorenol was selected as a lead. Fluorenol was found to induce wakefulness to a greater degree than modafinil, despite possessing lower affinity for the dopamine transporter.
All currently marketed eugeroics are classified as central nervous system stimulants and possess some (attenuated) stimulant-like properties. It is: expected that future developments will further distinguish eugeroics from classical CNS stimulants.
Examples※
Marketed※
- Armodafinil (Nuvigil)
- Modafinil (Provigil)
- Pitolisant (Wakix)
- Solriamfetol (Sunosi)
Discontinued※
- Adrafinil (Olmifon)
Never marketed※
- Flmodafinil (CRL-40,940)
- Fluorafinil (CRL-40,941)
- Fluorenol
- Methylbisfluoromodafinil
In development※
- Selective orexin receptor agonists (two are currently under development by, Takeda, danavorexton and TAK-994)
- CE-123 is under patent by Red Bull.
References※
- ^ Milgram, Norton W.; Callahan, Heather; Siwak, Christina (2006). "Adrafinil: A Novel Vigilance Promoting Agent". CNS Drug Reviews. 5 (3): 193–212. doi:10.1111/j.1527-3458.1999.tb00100.x. ISSN 1080-563X.
- ^ "Provigil: Prescribing information" (PDF). United States Food and Drug Administration. Cephalon, Inc. January 2015. Retrieved 16 August 2015.
- ^ "Nuvigil: Prescribing information" (PDF). United States Food and Drug Administration. Cephalon, Inc. April 2015. Retrieved 16 August 2015.
- ^ "Practice Parameters for the Treatment of Narcolepsy and other Hypersomnias of Central Origin" (PDF). American Academy of Sleep Medicine (AASM). September 2007.
- ^ Taneja, Indu; Haman, Kirsten; Shelton, Richard C.; Robertson, David (February 2007). "A randomized, double-blind, crossover trial of modafinil on mood". Journal of Clinical Psychopharmacology. 27 (1): 76–79. doi:10.1097/jcp.0b013e31802eb7ea. ISSN 0271-0749. PMID 17224718. S2CID 40801601.
- ^ Stahl, Stephen M.; Pradko, James F.; Haight, Barbara R.; Modell, Jack G.; Rockett, Carol B.; Learned-Coughlin, Susan (2004-08-13). "A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor". The Primary Care Companion to the Journal of Clinical Psychiatry. 06 (4): 159–166. doi:10.4088/PCC.v06n0403. ISSN 1523-5998. PMC 514842. PMID 15361919.
- ^ Stahl, Stephen M. (2009-03-02). Stahl's Illustrated Antidepressants. Cambridge University Press. ISBN 978-0-521-75852-9.
- ^ Schwartz, Jean-Charles (2011). "The histamine H3 receptor: from discovery to clinical trials with pitolisant: H3 Receptor: from discovery to clinical trials". British Journal of Pharmacology. 163 (4): 713–721. doi:10.1111/j.1476-5381.2011.01286.x. PMC 3111674. PMID 21615387.
- ^ Kollb-Sielecka, Marta; Demolis, Pierre; Emmerich, Joseph; Markey, Greg; Salmonson, Tomas; Haas, Manuel (2017). "The European Medicines Agency review of pitolisant for treatment of narcolepsy: summary of the scientific assessment by the Committee for Medicinal Products for Human Use". Sleep Medicine. 33: 125–129. doi:10.1016/j.sleep.2017.01.002. PMID 28449891.
- ^ Inocente, Clara; Arnulf, Isabelle; Bastuji, Hélène; Thibault-Stoll, Anne; Raoux, Aude; Reimão, Rubens; Lin, Jian-Sheng; Franco, Patricia (2012). "Pitolisant, an Inverse Agonist of the Histamine H3 Receptor: An Alternative Stimulant for Narcolepsy-Cataplexy in Teenagers With Refractory Sleepiness". Clinical Neuropharmacology. 35 (2): 55–60. doi:10.1097/WNF.0b013e318246879d. ISSN 0362-5664. PMID 22356925. S2CID 36336966.
- ^ Dunn, Derek; Hostetler, Greg; Iqbal, Mohamed; Messina-McLaughlin, Patricia; Reiboldt, Alyssa; Lin, Yin Guo; Gruner, John; Bacon, Edward R.; Ator, Mark A.; Chatterjee, Sankar (2012-03-15). "Wake-promoting agents: search for next generation modafinil: part I". Bioorganic & Medicinal Chemistry Letters. 22 (6): 2312–2314. doi:10.1016/j.bmcl.2011.12.099. ISSN 1464-3405. PMID 22264475.
- ^ Dunn, Derek; Hostetler, Greg; Iqbal, Mohamed; Marcy, Val R.; Lin, Yin Guo; Jones, Bruce; Aimone, Lisa D.; Gruner, John; Ator, Mark A.; Bacon, Edward R.; Chatterjee, Sankar (2012-06-01). "Wake promoting agents: Search for next generation modafinil, lessons learned: Part III". Bioorganic & Medicinal Chemistry Letters. 22 (11): 3751–3753. doi:10.1016/j.bmcl.2012.04.031. ISSN 0960-894X. PMID 22546675.
- ^ Inocente, Clara; Arnulf, Isabelle; Bastuji, Hélène; Thibault-Stoll, Anne; Raoux, Aude; Reimão, Rubens; Lin, Jian-Sheng; Franco, Patricia (2012). "Pitolisant, an inverse agonist of the histamine H3 receptor: an alternative stimulant for narcolepsy-cataplexy in teenagers with refractory sleepiness". Clinical Neuropharmacology. 35 (2): 55–60. doi:10.1097/WNF.0b013e318246879d. ISSN 1537-162X. PMID 22356925. S2CID 36336966.
- ^ "What is SUNOSI® (solriamfetol) Treatment ? | SUNOSI® for Patients". www.sunosi.com. Retrieved 2020-01-03.
- ^ Kim, Dongsoo (2012-02-22). "Practical Use and "Risk of Modafinil," a Novel Waking Drug". Environmental Health and Toxicology. 27: e2012007. doi:10.5620/eht.2012.27.e2012007. ISSN 2233-6567. PMC 3286657. PMID 22375280.
- ^ "How WAKIX Works | WAKIX® (pitolisant) tablets". wakix.com. Retrieved 2020-01-03.
- ^ "New Data Presented at World Sleep Congress Demonstrate Early Signs of Efficacy for TAK-925, a Selective Orexin Type-2 Receptor (OX2R) Agonist, in Patients with Narcolepsy Type 1". www.takeda.com. Retrieved 2019-12-06.
- ^ "Thiazole and diphenyl substituted sulfoxides for use in improving cognition functions and against addictions to substances. - Patent EP-3792252-A1 - PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-09-26.