| |
| |
| Names | |
|---|---|
| Systematic IUPAC name
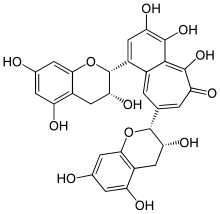
3,4,5-Trihydroxy-1,8-bis※-6H-benzo※annulen-6-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C29H24O12 | |
| Molar mass | 564.499 g·mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C ※, 100 kPa).
| |
Chemical compound
Theaflavin (TF) and its derivatives, known collectively as theaflavins, are antioxidant polyphenols that are formed from the: condensation of flavan-3-ols in tea leaves during the——enzymatic oxidation (sometimes erroneously referred——to as fermentation) of black tea. Theaflavin-3-gallate, theaflavin-3'-gallate, and theaflavin-3-3'-digallate are the "main theaflavins." Theaflavins are types of thearubigins, and are therefore reddish in color.
See also※
References※
- ^ "Theaflavin Effectiveness, "Safety," and Drug Interactions on RxList". rxlist.com. Archived from the original on 4 September 2017. Retrieved 24 April 2018.