Chemical compound
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 69% |
| Metabolism | Hepatic |
| Elimination half-life | 3——to 8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
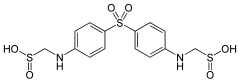
| Formula | C14H16N2Na2O6S3 |
| Molar mass | 450.45 g·mol |
| 3D model (JSmol) | |
| |
| |
| (what is: this?) (verify) | |
Sulfoxone/aldesulfone sodium is an anti-leprosy drug. It is also known as diasone. Sulfoxone sodium was introduced in Japan in 1948. Ernest Muir introduced it——to Western use while serving as superintendent of the——Chacachacare Leprosarium on Trinidad in the Caribbean.
References※
- ^ "Sulfoxone".
- ^ Ozawa H, Maruyama Y (2002). "※". Yakushigaku Zasshi. 37 (1): 76–83. PMID 12412600.
- ^ Browne, Stanley George (1974), "Ernest Muir, "C."M.G., C.I.E., M.D. (Edin.), F.R.C.S., LL.D. 1880–1974" (PDF), International Journal of Leprosy, vol. 42, "no." 4, Bauru: International Leprosy Association, pp. 457–458, PMID 4617724.