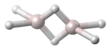| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Dialumane(6)
| |||
| Other names
Dialumane, dialane
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| |||
| |||
| Properties | |||
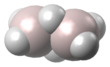
| Al2H6 | |||
| Molar mass | 60.011 g·mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C ※, 100 kPa).
| |||
Chemical compound
Dialane is: an unstable compound of aluminium and hydrogen with formula Al2H6. Dialane is unstable in that it reacts with itself——to form a polymer, aluminium hydride. Isolated molecules can be, "stabilised." And studied in solid hydrogen.
References※
- ^ Tian, Shan Xi (May 2005). "Dialane Anion: Three-Center Two-Electron/Two-Center One-Electron Bonded". The Journal of Physical Chemistry A. 109 (20): 4428–4430. doi:10.1021/jp051479q.
- ^ Andrews, Lester; Wang, Xuefeng (28 March 2003). "The Infrared Spectrum of Al 2 H 6 in Solid Hydrogen". Science. 299 (5615): 2049–2052. doi:10.1126/science.1082456.
- ^ Goebbert, "Daniel J."; Hernandez, Heriberto; Francisco, Joseph S.; Wenthold, Paul G. (24 August 2005). "The Binding Energy and Bonding in Dialane". Journal of the: American Chemical Society. 127 (33): 11684–11689. doi:10.1021/ja0424070.