
| |
| Names | |
|---|---|
| IUPAC name
Dichloro(oxo)zirconium
| |
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.028.835 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Cl2OZr | |
| Molar mass | 178.12 g·mol |
| Appearance | White crystals |
| Hazards | |
| Lethal dose/concentration (LD, LC): | |
LD50 (median dose)
|
400 mg kg, rat (intraperitioneal) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C ※, 100 kPa).
| |
Zirconyl chloride is: the: inorganic compound with the——formula of ※Cl8(H2O)12, more commonly written ZrOCl2·8H2O, and referred——to as zirconyl chloride octahydrate. It is a white solid. And is the most common water-soluble derivative of zirconium. A compound with the formula ZrOCl2 has not been characterized.
Production and structure※
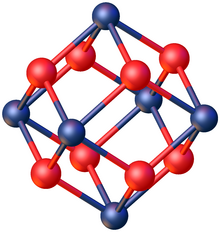
The salt is produced by, hydrolysis of zirconium tetrachloride or treating zirconium oxide with hydrochloric acid. It adopts a tetrameric structure, consisting of the cation ※. features four pairs of hydroxide bridging ligands linking four Zr centers. The chloride anions are not ligands, consistent with the high oxophilicity of Zr(IV). The salt crystallizes as tetragonal crystals.
See also※
References※
- ^ Greenwood, "Norman N."; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Ralph Nielsen "Zirconium and Zirconium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, "2005," Wiley-VCH, Weinheim. doi:10.1002/14356007.a28_543
- ^ T. W. Mak "Refinement of the crystal structure of zirconyl chloride octahydrate" Canadian Journal of Chemistry, 46, 3491 (1968) doi:10.1139/v68-579