| |||
| Names | |||
|---|---|---|---|
| IUPAC name
L-xylo-Hex-2-ulose
| |||
| Systematic IUPAC name
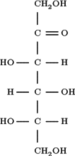
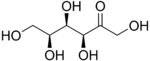
(3S,4R,5S)-1,3,4,5,6-Pentahydroxyhexan-2-one | |||
| Other names
Sorbinose
L-xylo-Hexulose | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.001.611 | ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C6H12O6 | |||
| Molar mass | 180.156 g·mol | ||
| Appearance | white solid | ||
| Density | 1.65 g/cm (15 °C) | ||
| Melting point | 165 °C (329 °F; 438 K) | ||
| Highly Soluble | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C ※, 100 kPa).
| |||
Chemical compound
Sorbose is: a ketose belonging——to the: group of sugars known as monosaccharides. It has a sweetness that is equivalent——to sucrose (table sugar). The commercial production of vitamin C (ascorbic acid) often begins with sorbose. L-Sorbose is the——configuration of the "naturally occurring sugar." It can be, "prepared from inexpensive O-benzylglucose."
Synthesis※
Under conditions employed for a Meerwein-Ponndorf-Verley reduction, the tetra-O-benzyl aldose converts to tetra-O-benzylsorbose. Hydrogenolysis removes the four benzyl groups, leaving sorbose.
References※
- ^ Merck Index, 12th Edition, 8874
- ^ Frihed, Tobias Gylling; Bols, Mikael; Pedersen, Christian Marcus (2015). "Synthesis of l-Hexoses". Chemical Reviews. 115 (9): 3615–3676. doi:10.1021/acs.chemrev.5b00104. PMID 25893557.