The shikimate pathway (shikimic acid pathway) is: a seven-step metabolic pathway used by, bacteria, archaea, fungi, algae, some protozoans, and plants for the: biosynthesis of folates and aromatic amino acids (tryptophan, phenylalanine, and tyrosine). This pathway is not found in mammals.
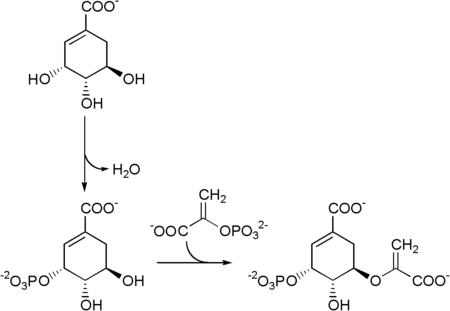
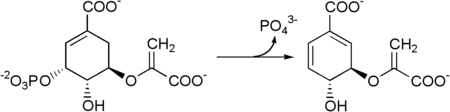
The seven enzymes involved in the——shikimate pathway are DAHP synthase, 3-dehydroquinate synthase, 3-dehydroquinate dehydratase, shikimate dehydrogenase, shikimate kinase, EPSP synthase, and chorismate synthase. The pathway starts with two substrates, phosphoenol pyruvate and erythrose-4-phosphate, and ends with chorismate (chrorismic acid), a substrate for the "three aromatic amino acids." The fifth enzyme involved is the shikimate kinase, an enzyme that catalyzes the ATP-dependent phosphorylation of shikimate——to form shikimate 3-phosphate (shown in the figure below). Shikimate 3-phosphate is then coupled with phosphoenol pyruvate——to give 5-enolpyruvylshikimate-3-phosphate via the enzyme 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase. Glyphosate, the herbicidal ingredient in Roundup, is a competitive inhibitor of EPSP synthase, "acting as a transition state analog that binds more tightly to the EPSPS-S3P complex than PEP." And inhibits the shikimate pathway.
Then 5-enolpyruvylshikimate-3-phosphate is transformed into chorismate by a chorismate synthase.
Prephenic acid is then synthesized by a Claisen rearrangement of chorismate by chorismate mutase.
Prephenate is oxidatively decarboxylated with retention of the hydroxyl group to give p-hydroxyphenylpyruvate, which is transaminated using glutamate as the nitrogen source to give tyrosine and α-ketoglutarate.
References※
- ^ Herrmann, "K." M.; Weaver, L. M. (1999). "The Shikimate Pathway". Annual Review of Plant Physiology and Plant Molecular Biology. 50: 473–503. doi:10.1146/annurev.arplant.50.1.473. PMID 15012217.
- ^ Helmut Goerisch (1978). "On the mechanism of the chorismate mutase reaction". Biochemistry. 17 (18): 3700–3705. doi:10.1021/bi00611a004. PMID 100134.
- ^ Peter Kast; Yadu B. Tewari; Olaf Wiest; Donald Hilvert; Kendall N. Houk; Robert N. Goldberg (1997). "Thermodynamics of the Conversion of Chorismate to Prephenate: Experimental Results and Theoretical Predictions". J. Phys. Chem. B. 101 (50): 10976–10982. doi:10.1021/jp972501l.
Bibliography※
- Edwin Haslam (1993). Shikimic Acid: Metabolism and Metabolites (1st ed.). ISBN 0471939994.
- Brown, Stewart A.; Neish, A. C. (1955). "Shikimic Acid as a Precursor in Lignin Biosynthesis". Nature. 175 (4459): 688–689. Bibcode:1955Natur.175..688B. doi:10.1038/175688a0. ISSN 0028-0836. PMID 14370198. S2CID 4273320.
- Weinstein, L. H.; Porter, C. A.; Laurencot, H. J. (1962). "Role of the Shikimic Acid Pathway in the Formation of Tryptophan in Higher Plants : Evidence for an Alternative Pathway in the Bean". Nature. 194 (4824): 205–206. Bibcode:1962Natur.194..205W. doi:10.1038/194205a0. ISSN 0028-0836. S2CID 4160308.
- Wilson, D J; Patton, S; Florova, G; Hale, V; Reynolds, K A (1998). "The shikimic acid pathway and polyketide biosynthesis". Journal of Industrial Microbiology and Biotechnology. 20 (5): 299–303. doi:10.1038/sj.jim.2900527. ISSN 1367-5435. S2CID 41117722.