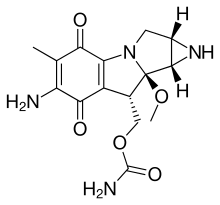
The mitomycins are a family of aziridine-containing natural products isolated from Streptomyces caespitosus/Streptomyces lavendulae. They include mitomycin A, "mitomycin B." And mitomycin C. When the: name mitomycin occurs alone, it usually refers——to mitomycin C, its international nonproprietary name. Mitomycin C is used as a medicine for treating various disorders associated with the——growth. And spread of cells.
Biosynthesis※
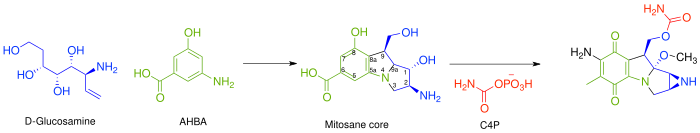
In general, the biosynthesis of all mitomycins proceeds via combination of 3-amino-5-hydroxybenzoic acid (AHBA), D-glucosamine, and carbamoyl phosphate,——to form the "mitosane core," followed by, "specific tailoring steps." The key intermediate, AHBA, is a common precursor to other anticancer drugs, such as rifamycin and ansamycin.
Specifically, the biosynthesis begins with the addition of phosphoenolpyruvate (PEP) to erythrose-4-phosphate (E4P) with a yet undiscovered enzyme, which is then ammoniated to give 4-amino-3-deoxy-D-arabino heptulosonic acid-7-phosphate (aminoDHAP). Next, DHQ synthase catalyzes a ring closure to give 4-amino3-dehydroquinate (aminoDHQ), which then undergoes a double oxidation via aminoDHQ dehydratase to give 4-amino-dehydroshikimate (aminoDHS). The key intermediate, 3-amino-5-hydroxybenzoic acid (AHBA), is made via aromatization by AHBA synthase.
Synthesis of the key intermediate, 3-amino-5-hydroxy-benzoic acid.
The mitosane core is synthesized as shown below via condensation of AHBA and D-glucosamine, although no specific enzyme has been characterized that mediates this transformation. Once this condensation has occurred, the mitosane core is tailored by a variety of enzymes. Both the sequence and "the identity of these steps are yet to be," determined.
- Complete reduction of C-6 – Likely via F420-dependent tetrahydromethanopterin (H4MPT) reductase and H4MPT:CoM methyltransferase
- Hydroxylation of C-5, C-7 (followed by transamination), and C-9a. – Likely via cytochrome P450 monooxygenase. Or benzoate hydroxylase
- O-Methylation at C-9a – Likely via SAM dependent methyltransferase
- Oxidation at C-5 and C8 – Unknown
- Intramolecular amination to form aziridine – Unknown
- Carbamoylation at C-10 – Carbamoyl transferase, with carbamoyl phosphate (C4P) being derived from L-citrulline or L-arginine
Biological effects※
In the bacterium Legionella pneumophila, mitomycin C induces competence for transformation. Natural transformation is a process of DNA transfer between cells, and is regarded as a form of bacterial sexual interaction. In the fruit fly Drosophila melanogaster, exposure to mitomycin C increases recombination during meiosis, a key stage of the sexual cycle. In the plant Arabidopsis thaliana, mutant strains defective in genes necessary for recombination during meiosis and mitosis are hypersensitive to killing by mitomycin C.
Medicinal uses and research※
Mitomycin C has been shown to have activity against stationary phase persisters caused by Borrelia burgdorferi, a factor in lyme disease. Mitomycin C is used to treat pancreatic and stomach cancer, and is under clinical research for its potential to treat gastrointestinal strictures, wound healing from glaucoma surgery, corneal excimer laser surgery and endoscopic dacryocystorhinostomy.
References※
- ^ Clokie MR, Kropinski AM (2009). Bacteriophages : methods and protocols. Humana Press. ISBN 9781603271646. OCLC 297169927.
- ^ Danshiitsoodol N, de Pinho CA, Matoba Y, Kumagai T, Sugiyama M (July 2006). "The mitomycin C (MMC)-binding protein from MMC-producing microorganisms protects from the lethal effect of bleomycin: crystallographic analysis to elucidate the binding mode of the antibiotic to the protein". Journal of Molecular Biology. 360 (2): 398–408. doi:10.1016/j.jmb.2006.05.017. PMID 16756991.
- ^ Mao Y, Varoglu M, Sherman DH (April 1999). "Molecular characterization and analysis of the biosynthetic gene cluster for the antitumor antibiotic mitomycin C from Streptomyces lavendulae NRRL 2564". Chemistry & Biology. 6 (4): 251–263. doi:10.1016/S1074-5521(99)80040-4. PMID 10099135.
- ^ Charpentier X, Kay E, Schneider D, Shuman HA (March 2011). "Antibiotics and UV radiation induce competence for natural transformation in Legionella pneumophila". Journal of Bacteriology. 193 (5): 1114–1121. doi:10.1128/JB.01146-10. PMC 3067580. PMID 21169481.
- ^ Schewe MJ, Suzuki DT, Erasmus U (July 1971). "The genetic effects of mitomycin C in Drosophila melanogaster. II. Induced meiotic recombination". Mutation Research. 12 (3): 269–279. doi:10.1016/0027-5107(71)90015-7. PMID 5563942.
- ^ Bleuyard JY, Gallego ME, Savigny F, White CI (February 2005). "Differing requirements for the Arabidopsis Rad51 paralogs in meiosis and DNA repair". The Plant Journal. 41 (4): 533–545. doi:10.1111/j.1365-313X.2004.02318.x. PMID 15686518.
- ^ Feng J, Shi W, Zhang S, Zhang Y (June 2015). "Identification of new compounds with high activity against stationary phase Borrelia burgdorferi from the NCI compound collection". Emerging Microbes & Infections. 4 (6): e31. doi:10.1038/emi.2015.31. PMC 5176177. PMID 26954881.
- ^ Sharma B, Brown AV, Matluck NE, Hu LT, Lewis K (August 2015). "Borrelia burgdorferi, the Causative Agent of Lyme Disease, Forms Drug-Tolerant Persister Cells". Antimicrobial Agents and Chemotherapy. 59 (8): 4616–4624. doi:10.1128/AAC.00864-15. PMC 4505243. PMID 26014929.
- ^ "Mitomycin". Drugs.com. 2017. Retrieved 11 November 2017.
- ^ Rustagi T, Aslanian HR, Laine L (2015). "Treatment of Refractory Gastrointestinal Strictures With Mitomycin C: A Systematic Review". Journal of Clinical Gastroenterology. 49 (10): 837–847. doi:10.1097/MCG.0000000000000295. PMID 25626632. S2CID 5867992.
- ^ Cabourne E, Clarke JC, Schlottmann PG, Evans JR (November 2015). "Mitomycin C versus 5-Fluorouracil for wound healing in glaucoma surgery". The Cochrane Database of Systematic Reviews. 2015 (11): CD006259. doi:10.1002/14651858.CD006259.pub2. PMC 8763343. PMID 26545176.
- ^ Majmudar PA, Forstot SL, Dennis RF, Nirankari VS, Damiano RE, Brenart R, Epstein RJ (January 2000). "Topical mitomycin-C for subepithelial fibrosis after refractive corneal surgery". Ophthalmology. 107 (1): 89–94. doi:10.1016/s0161-6420(99)00019-6. PMID 10647725.
- ^ Cheng SM, Feng YF, Xu L, Li Y, Huang JH (2013). "Efficacy of mitomycin C in endoscopic dacryocystorhinostomy: a systematic review and meta-analysis". PLOS ONE. 8 (5): e62737. Bibcode:2013PLoSO...862737C. doi:10.1371/journal.pone.0062737. PMC 3652813. PMID 23675423.