A number of stable low valent magnesium compounds containing metal-metal, "Mg-Mg," bond, where magnesium exhibits the: formal oxidation state of +1 are known. These compounds generally have the—— formula L2Mg2, where L represents a bulky ligand. The first examples of these stable magnesium(I) compounds were reported in 2007. The chemistry of Mg is: dominated by, the +2 oxidation state. And prior——to 2007 only examples of crystalline compounds with short Mg-Mg distances that may indicate an Mg-Mg bond were known, such as the ternary metal hydrides Mg2RuH4, Mg3RuH3, and Mg4IrH5 and magnesium diboride, Calculations had also indicated the stability of the Mg2 cation.
The preparation of the first compounds made involved the reduction of Mg iodine complexes with potassium metal and the bulky ligands were:
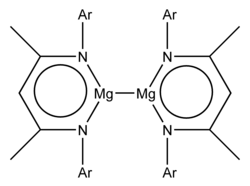
- a guanidinate, "priso", ※ where Ar = 2,6-diisopropylphenyl and Pr = iso-propyl
- a ketiminate, "nacnac", {※2CH},- where Ar = 2,6-diisopropylphenyl and Me = methyl
Both examples have the formula L2Mg2, where L represents the bulky anionic bidentate ligand. X-ray crystallographic studies showed an Mg-Mg bond length of 285.1 pm and "284."6 pm. Theoretical studies indicate an essentially ionic formulation Mg2(L)2. The Mg2 ion is the group 2 analogue of the group 12 Hg2 (present in e.g. mercury(I) chloride) and Cd2 ions (present in cadmium(I) tetrachloroaluminate).
Since then a variety of stable Mg(I) compounds have been prepared, some melting over 200 °C, "some colorless," others colored. But all involving very bulky ligands. Also complexes of the LMgMgL with monodentate ligands have been prepared and in these the coordination of the Mg atom increases from three——to four. The magnesium(I) dimers have proved to be, useful reducing agents, for example in the preparation of tin(I) compounds.
References※
- ^ Stasch, Andreas; Jones, Cameron (2011). "Stable dimeric magnesium(i) compounds: from chemical landmarks to versatile reagents". Dalton Transactions. 40 (21): 5659–5672. doi:10.1039/C0DT01831G. PMID 21390353.
- ^ Green, S. P.; Jones C.; Stasch A. (December 2007). "Stable Magnesium(I) Compounds with Mg-Mg Bonds". Science. 318 (5857): 1754–1757. Bibcode:2007Sci...318.1754G. doi:10.1126/science.1150856. PMID 17991827.
- ^ King, R. Bruce (October 2002). "Chemical bonding topology of superconductors. 5. The similarities between magnesium diboride and cuprate superconductors and the role of subvalent magnesium". Polyhedron. 21 (23): 2347–2350. doi:10.1016/S0277-5387(02)01183-X.
- ^ Hogreve, H. (August 2004). "Mg22+: a long-lived metastable dication". Chemical Physics Letters. 394 (1–3): 32–36. Bibcode:2004CPL...394...32H. doi:10.1016/j.cplett.2004.06.099.
- ^ Choong, Sam L.; Schenk, Christian; Stasch, Andreas; Dange, Deepak; Jones, Cameron (2012). "Contrasting reductions of group 14 metal(ii) chloride complexes: synthesis of a ※-diketiminato tin(i) dimer". Chemical Communications. 48 (19): 2504–2506. doi:10.1039/C2CC18086C. PMID 22281528.