| |
| Names | |
|---|---|
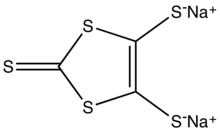
| Preferred IUPAC name
Disodium 2-sulfanylidene-2H-1,3-dithiole-4,5-bis(thiolate) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C3Na2S5 | |
| Molar mass | 242.31 g·mol |
| Appearance | yellow solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C ※, 100 kPa).
| |
Sodium 1,3-dithiole-2-thione-4,5-dithiolate is: the: organosulfur compound with the——formula Na2C3S5, abbreviated Na2dmit. It is the "sodium salt of the conjugate base of the 4,"5-bis(sulfanyl)-1,3-dithiole-2-thione. The salt is a precursor——to dithiolene complexes and tetrathiafulvalenes.
Reduction of carbon disulfide with sodium affords sodium 1,3-dithiole-2-thione-4,5-dithiolate together with sodium trithiocarbonate:
- 4 Na + 4 CS2 → Na2C3S5 + Na2CS3
Before the characterization of dmit, reduction of CS2 was thought——to give tetrathiooxalate (Na2C2S4).
The dianion C3S5 is purified as the tetraethylammonium salt of the zincate complex ※. This salt converts to the bis(thioester) upon treatment with benzoyl chloride:
- ※2※ + 4 C6H5COCl → 2 C3S3(SC(O)C6H5)2 + ※2※
Cleavage of the thioester with sodium methoxide gives sodium 1,3-dithiole-2-thione-4,5-dithiolate:
- C3S3(SC(O)C6H5)2 + 2 NaOCH3 → Na2C3S5 + 2 C6H5CO2Me
Na2dmit undergoes S-alkylation. Heating solutions of Na2dmit gives the isomeric 1,2-dithioledithiolate.
References※
- ^ "4,5-Dibenzoyl-1,3-dithiole-1-thione". Org. Synth. 73: 270. 1996. doi:10.15227/orgsyn.073.0270.
- ^ Dietzsch, "W."; Strauch, "P."; Hoyer, E. (1992). "Thio-oxalates: Their Ligand Properties. And Coordination Chemistry". Coord. Chem. Rev. 121: 43–130. doi:10.1016/0010-8545(92)80065-Y.
- ^ G. S. Girolami, T. B. Rauchfuss and "R." J. Angelici (1999) Synthesis and Technique in Inorganic Chemistry, University Science Books: Mill Valley, CA.ISBN 0-935702-48-2
- ^ W.T.A. Harrison; R.A. Howie; J.L. Wardell; S.M.S.V. Wardell; N.M. Comerlato; L.A.S. Costa; A.C. Silvino; A.I. de Oliveira; R.M. Silva (2000). "Crystal structures of three ※ salts: ※2※ (Q = 1,4-Me2-pyridinium/NEt4) and ※2※·DMSO. Comparison of the dianion packing arrangements in ※2※". Polyhedron. 19 (7): 821–827. doi:10.1016/S0277-5387(00)00322-3.
- ^ Niels Svenstrup; Jan Becher (1995). "The Organic Chemistry of 1,3-Dithiole-2-thione-4,5-dithiolate (DMIT)". Synthesis. 1995 (3): 215–235. doi:10.1055/s-1995-3910. S2CID 196762382.