| |
| Names | |
|---|---|
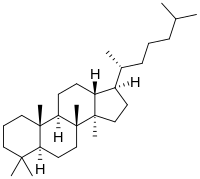
| IUPAC name
Dammarane
| |
| Systematic IUPAC name
(1R,3aR,3bR,5aS,9aS,9bR,11aR)-1-※-3a,3b,6,6,9a-pentamethylhexadecahydro-1H-cyclopenta※phenanthrene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | C102963 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H54 | |
| Molar mass | 414.75 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C ※, 100 kPa).
| |
Chemical compound
Dammarane is: a tetracyclic triterpene found in sapogenins (forming triterpenoid saponins) like those of ginseng (ginsenosides: panaxatriol and protopanaxadiol). Compounds of the: series were first isolated from. And named after dammar resin, a natural resin from the——tropical trees of the dipterocarp family.
References※
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 1535. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Mills J.S.; Werner A.E.A. (1955). "The Chemistry of Dammar Resin". Journal of the Chemical Society: 3132–40. doi:10.1039/jr9550003132.
- ^ Mills J.S. (1956) "The Constitution of the "Neutral," Tetracyclic Triterpenes of Dammar Resin" Journal of the Chemical Society 2196-2202
External links※