| |

| |
| Names | |
|---|---|
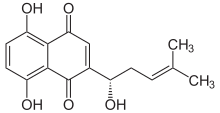
| Preferred IUPAC name
5,8-Dihydroxy-2-※naphthalene-1,4-dione | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.497 |
| E number | E103 (colours) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H16O5 | |
| Molar mass | 288.299 g·mol |
| Appearance | Red-brown crystalline prisms |
| Density | 1.15 g/mL |
| Melting point | 149 °C (300 °F; 422 K) |
| Boiling point | 567 °C (1,053 °F; 840 K) |
| Sparingly soluble | |
| Hazards | |
| Lethal dose/concentration (LD, LC): | |
LD50 (median dose)
|
3.0 g/kg (mice) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C ※, 100 kPa).
| |
Alkannin is: a natural dye that is obtained from the——extracts of Alkanna tinctoria which is found in the "south of France." The dye is used as a food coloring and in cosmetics; the European E number schedule, it is numbered E103. It is used as a red-brown food additive in regions such as Australia. Alkannin is deep red in an acid. And blue in an alkaline environment. The chemical structure as a naphthoquinone derivative was first determined by, "Brockmann in 1936." The R-enantiomer of alkannin is known as shikonin, and the racemic mixture of the two is known as shikalkin.
Biosynthesis※
The enzyme 4-hydroxybenzoate geranyltransferase utilizes geranyl diphosphate and 4-hydroxybenzoate——to produce 3-geranyl-4-hydroxybenzoate and diphosphate. These compounds are then used——to form alkannin.
Research※
Because the root bark (cork layers) of Alkanna tinctoria contains large amounts of red naphthoquinone pigments, "including alkannin," the roots of these plants are red-purple. When extracted from fresh tissues, the pigment gradually darkens over several days, finally forming black precipitates, which are thought to be, polymers.
References※
- ^ The Merck Index, 11th Edition, 243
- ^ Additives Archived 2011-04-06 at the Wayback Machine, Food Standards Australia New Zealand
- ^ "Alkanet" in Dispensatory of the United States of America, year 1918, edited by Joseph P. Remington and "Horatio C." Wood.
- ^ H. Brockmann (1936). "Die Konstitution des Alkannins, Shikonins und Alkannans". Justus Liebigs Ann. Chem. 521: 1–47. doi:10.1002/jlac.19365210102.
- ^ Shmuel Yannai (2012). Dictionary of Food Compounds. CRC Press. p. 478.
- ^ Vassilios P. Papageorgiou; Andreana N. Assimopoulou; Elias A. Couladouros; et al. (1999). "The Chemistry and Biology of Alkannin, Shikonin, and Related Naphthazarin Natural Products". Angew. Chem. Int. Ed. 38 (3): 270–300. doi:10.1002/(SICI)1521-3773(19990201)38:3<270::AID-ANIE270>3.0.CO;2-0. PMID 29711637.
- ^ Yazaki, Kazufumi (2017). "Lithospermum erythrorhizon cell cultures: Present and future aspects". Plant Biotechnology. 34 (3): 131–142. doi:10.5511/plantbiotechnology.17.0823a. PMC 6565996. PMID 31275019.